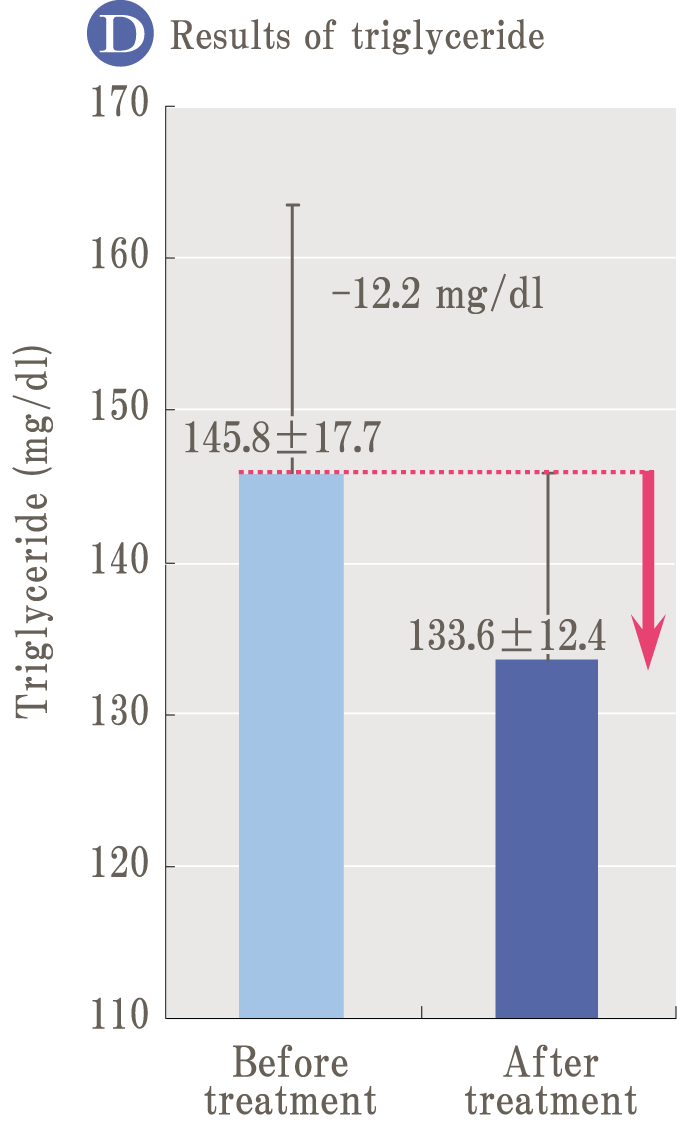
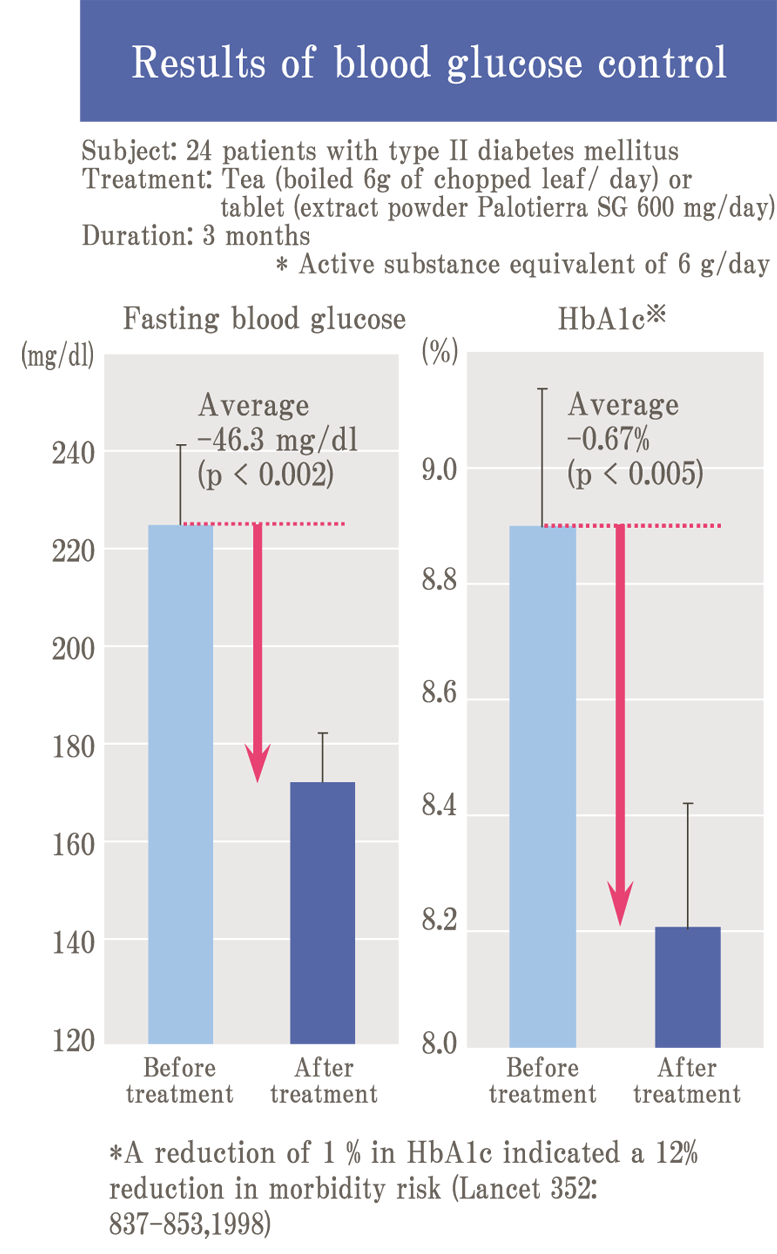
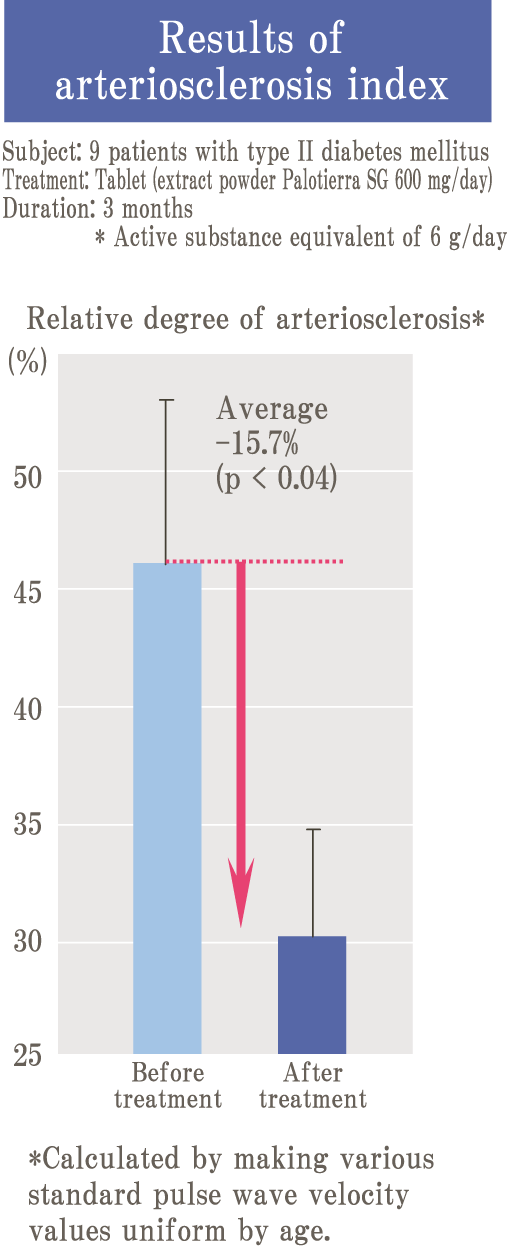
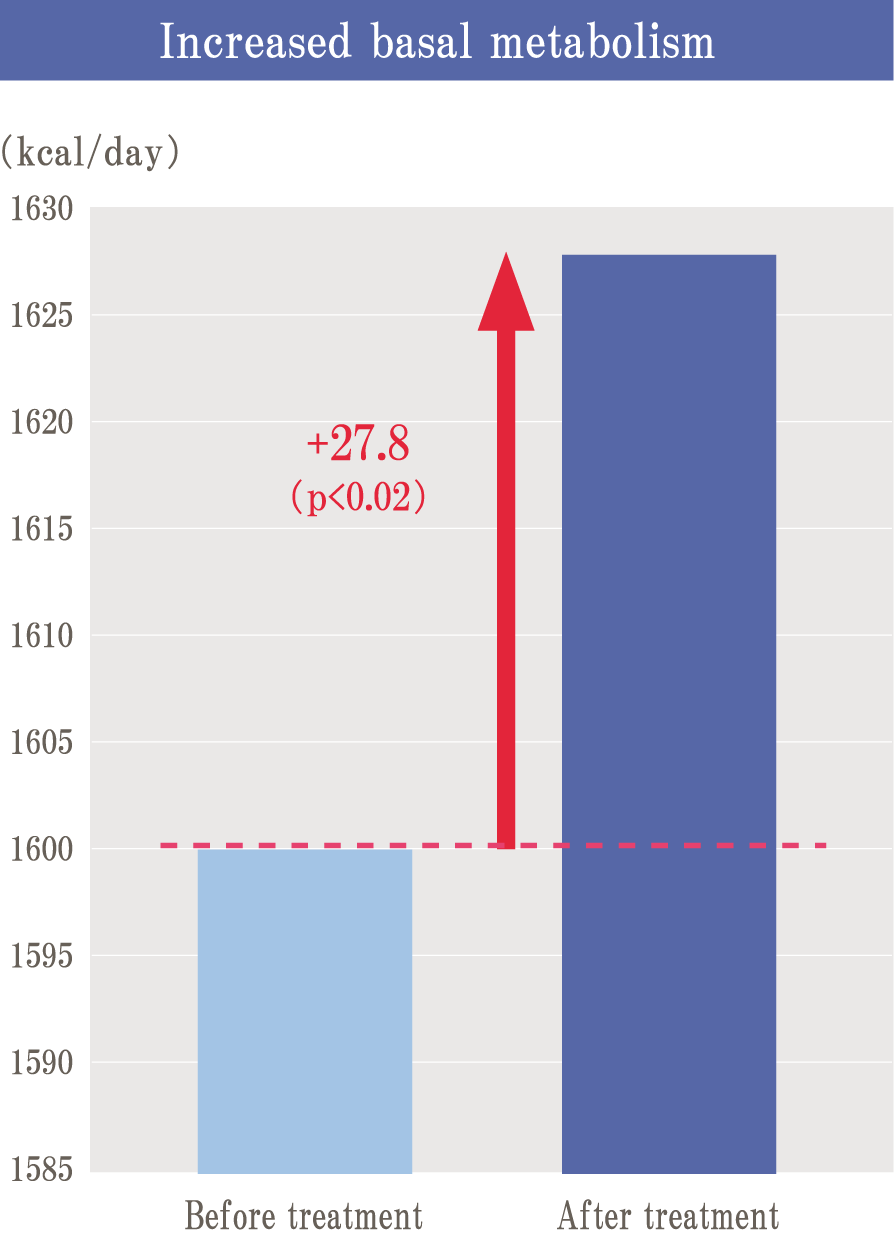
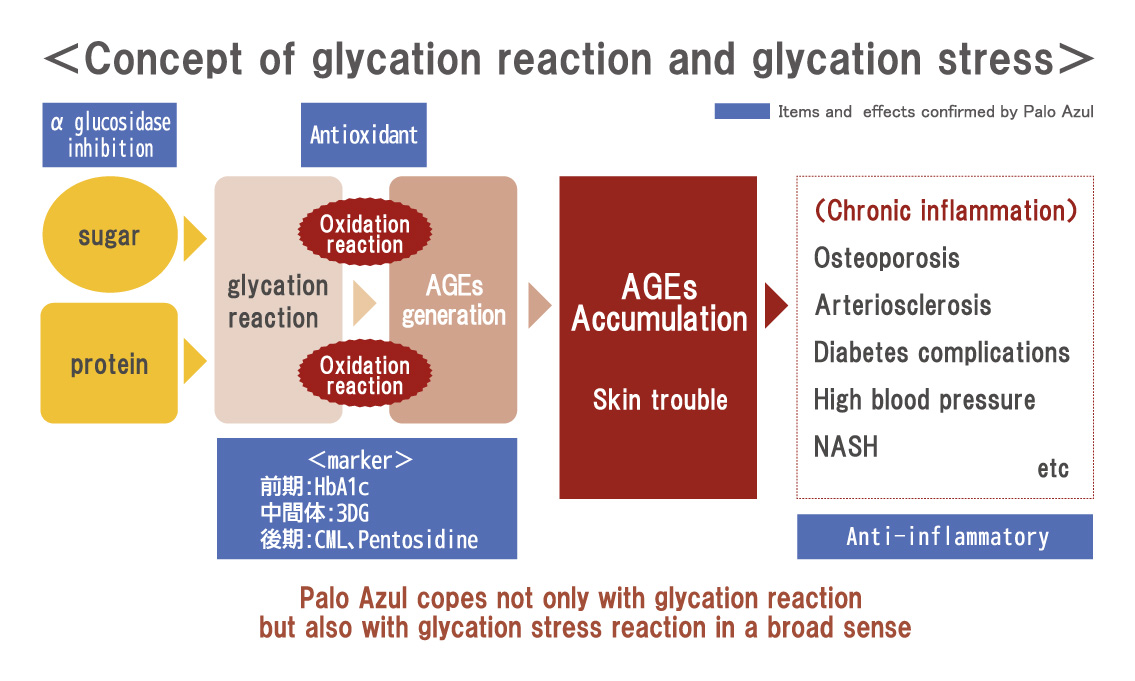
Glycation is a reaction (Maillard reaction) that turns brown mainly by combining sugar and protein, and it is also an important element related to taste in food.
However, when a glycation reaction occurs in the body, an aging substance called AGEs (final product of glycation) is produced and accumulated in various sites, which leads to systemic aging.
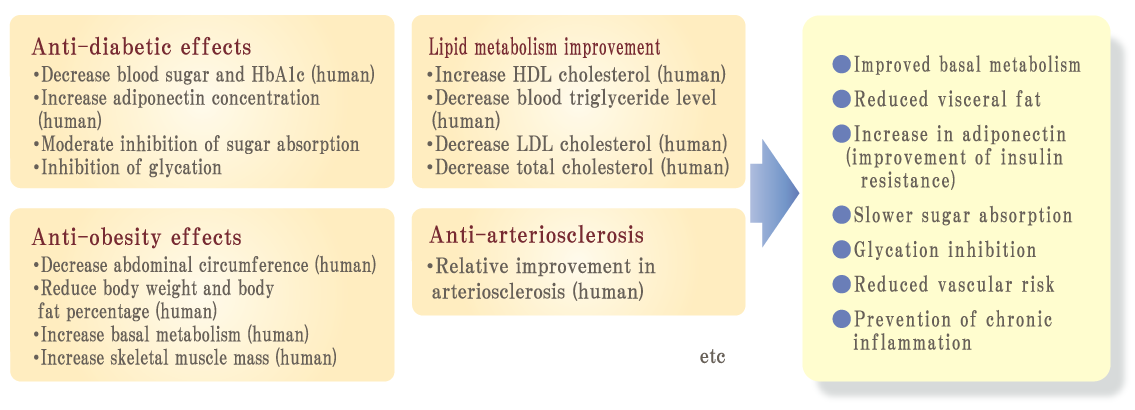
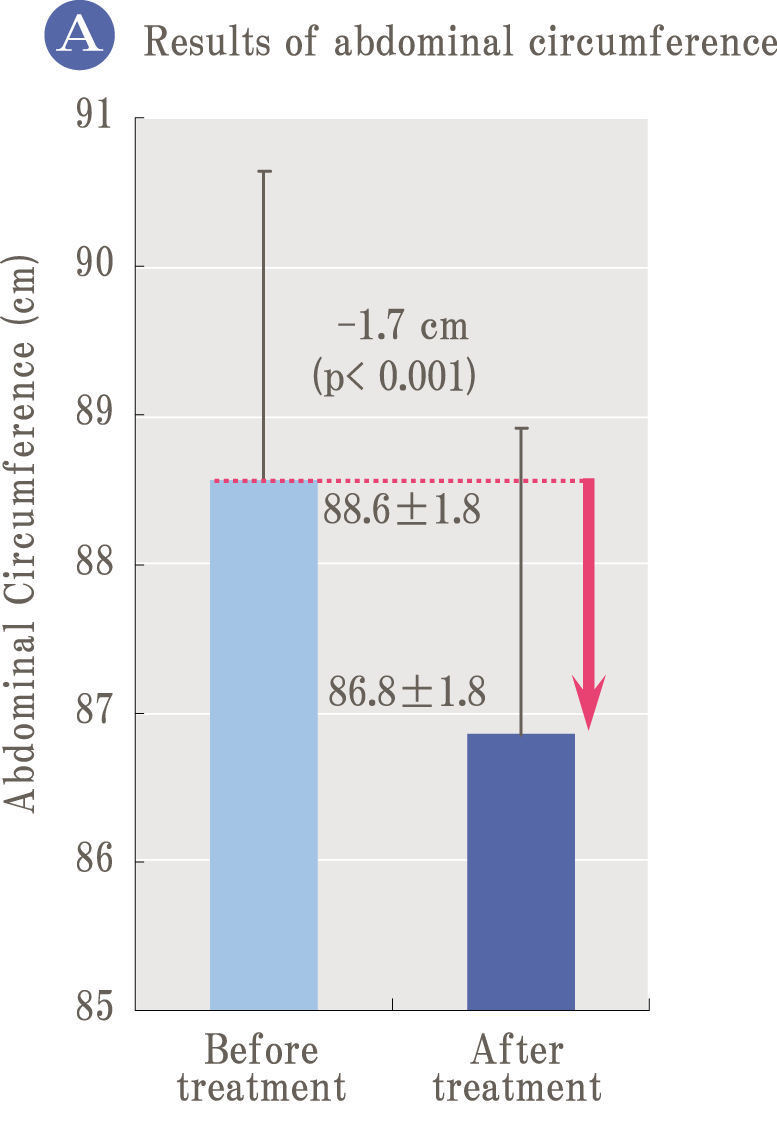
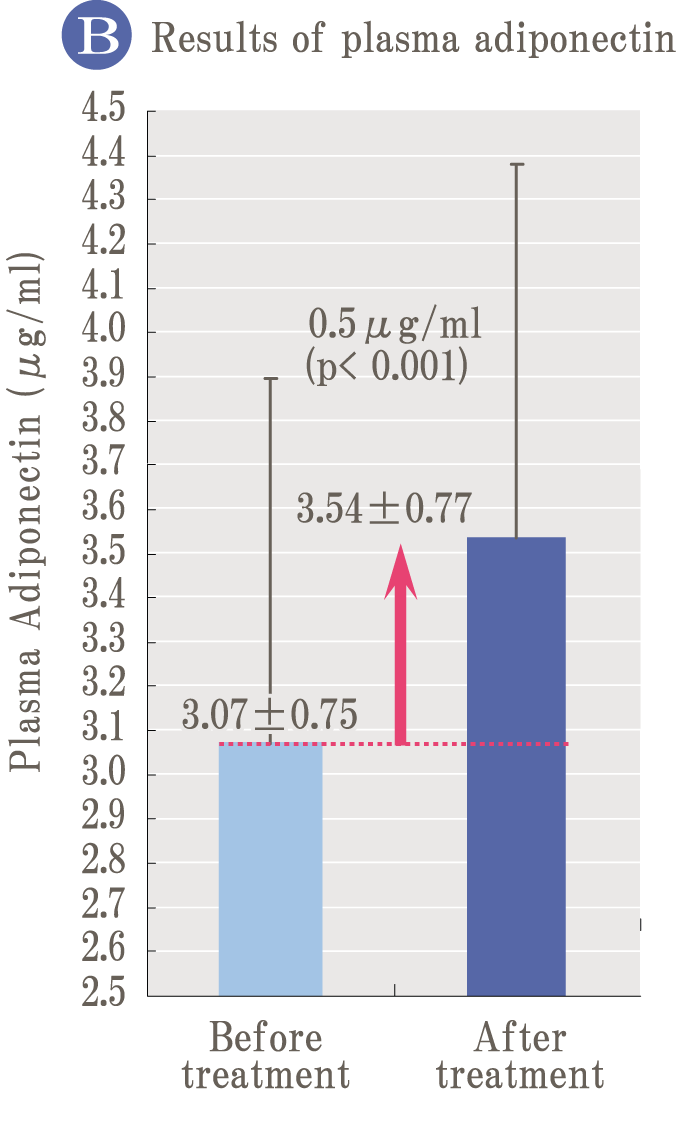
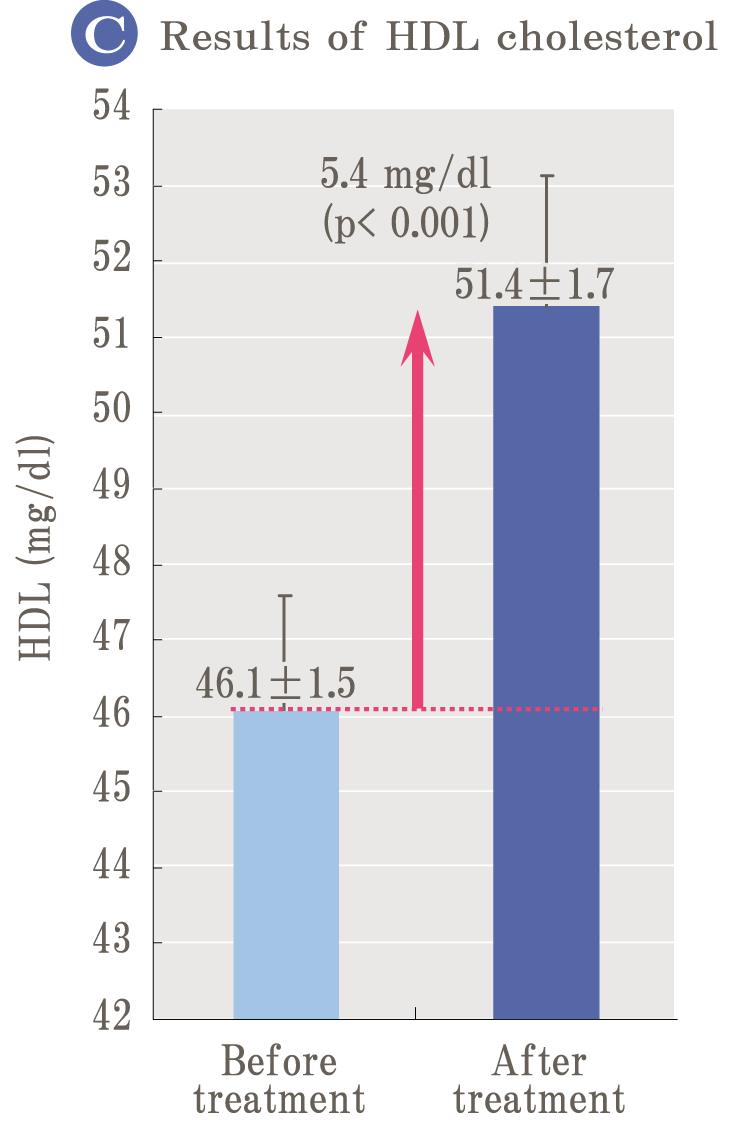
Because oxidation and glycation are related like two sides of a coin, it is important to prevent not only oxidation but also glycation simultaneously to prevent aging. Palo Azul supports not only glycation reaction but also general glycation stress reaction widely.
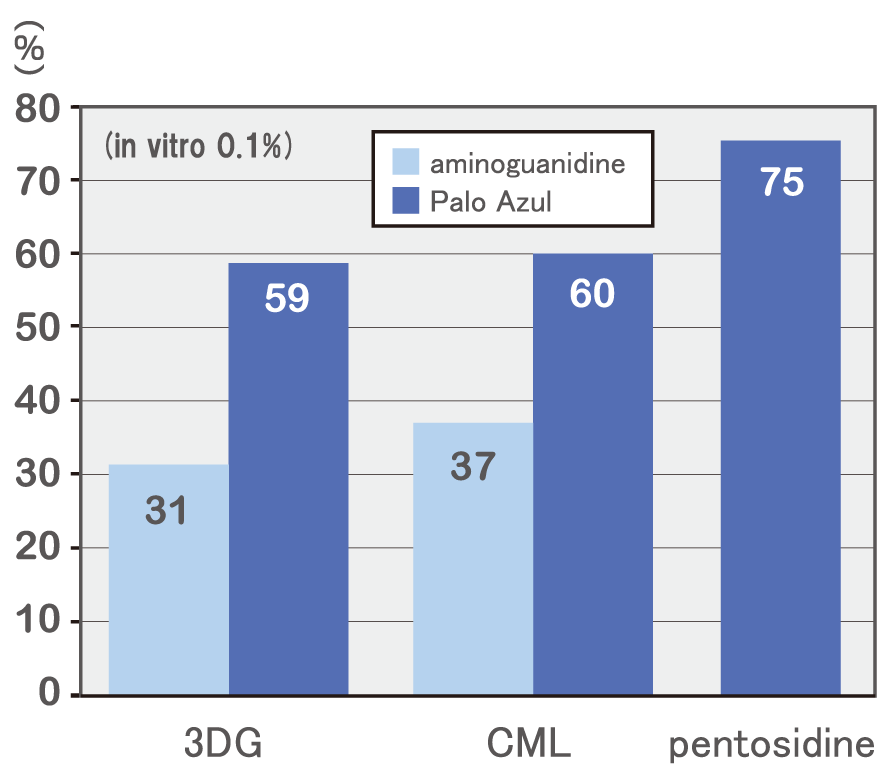
Effect of inhibition of glycation end products of Palo Azul extract
It was found that Palo Azul extract powder has 3DG production inhibitory action, pentosidine production inhibitory action and CML production inhibitory action. Furthermore, regarding 3DG and CML. Palo Azul extract powder shows higher inhibition rate than aminoguanidine (*).
*Aminoguanidine: A typical AGEs production inhibitor, which is a therapeutic drug for diabetic complications (not approved in Japan)
There is no measured value for pentosidine because no positive control is set.
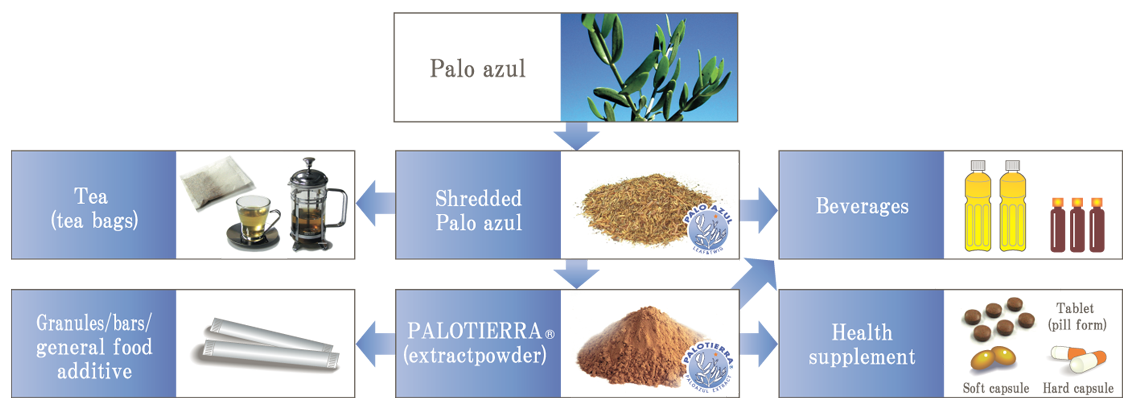
We supply the shredded tea leaf material and the alcohol extract powder named “Palotierra” as a raw material using Palo Azul.
“Palotierra” is a registered trademark of IHM Co., Ltd.
Product applications include tablets, capsules, granules, beverages, confectionery and general food applications.
Product specification etc.
Product name
For tea: Shredded Palo azul (roasted and sterilized)
Extra powder raw materials: PALOTIERRARSG (Palo azul extract powder with hydrous alcohol)
Country of origin: Republic of Paraguay
Appearance
For tea: Light brown-colored shreds with a characteristic odor
Extra powder raw materials: Light yellow-colored powder with a specific odor and salty taste
Recommended daily intake
For tea: 3g / Extra powder raw materials: 300-600mg
Shipping unit (packaging)
For tea: 1kg / Extra powder raw materials: 1kg
Safety studies (Tests conducted in Japan)
窶「Acute oral toxicity test 窶「Repeated dose 90-day toxicity study 窶「Mutagenicity test 窶「Residual pesticide 窶「Heavy metal 窶「Bacteria 窶「Moulds 窶「Yeasts, etc.
| Company name |
IHM Inc. |
| Headquarter |
2F,Ichigo Higashigotanda Bldg.,1-6-3 Higashigotanda Shinagawa-ku Tokyo 141-0022
Tel +81-3-4221-2207 |
| Capital |
98 million yen |
| Representative |
Shinji Irie, President |
| Business line |
Wholesale of pharmaceuticals, quasi-drugs, health food,
cosmetics, medical devices, etc. Sales of functional food materials,
Health food products planning and development |
| Number of employees |
30 |
| Partner banks |
Hokuriku Bank, MUFG Bank and Kiraboshi Bank |
| Major partners |
KOKANDO Co., Ltd.,
TOKIWA Pharmaceutical Co., Ltd.,
DAITO Pharmaceutical Co., Ltd,
GOSHU YAKUHIN CO., LTD. |
| Group sales company |
Hokkaido Medical Systems Inc.
Yamato Medical Inc.
Shutoken Medical Inc.
Chubu Medical Systems Inc.
Kansai Medical Systems Inc.
Nishinihon Medical Inc.
Kyushu Medical Inc.
Life Medical Inc. |
| Membership of Organization |
The Tokyo Chamber of Commerce and Industry |
Inquiry>


 日本語
日本語 簡体中文
簡体中文